Which Best Explains Why Water Dissolves Most Salts
The biggest similarity between these two substances is that their molecules are charged making them reactive. Salts are generally made up two ionically bonded atoms or molecules or more properly ions.

Why Does Water Dissolve Salt Youtube
Water consists of two hydrogen atoms and one oxygen molecule connected by a covalent bond to form a charged H20 molecule.

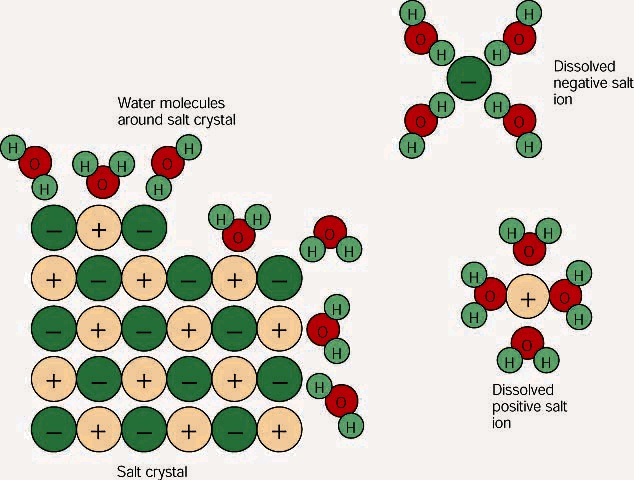
. The positive part of the water molecule the hydrogen part is attracted to the negative part of the salt the chlorine part. Water dissolves many biomolecules because they are polar and therefore hydrophilic. The positively-charged side of the water molecules are attracted to the negatively-charged chloride ions and the negatively-charged side of the water molecules are attracted to the positively-charged sodium ions.
Because water is a polar molecule each of its ends holds a slight positive or negative electrical charge. Salt dissolves in water because the water molecule has a negative and a positive end. At the molecular level salt dissolves in water due to electrical charges.
Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions and the negative part of water molecules attracts the positive sodium ions. Is this change physical or chemicalThis interactive explains. Why Dissolving Salt Is a Chemical Change.
Thus the ions are solvated hydrated. This is why salt dissolves in water. Raising the temperature increases the kinetic energy of the particles increasing the amount of salt that can be dissolved in the water.
Water molecules pulling apart the ions sodium and chloride in a salt crystal and then dissolving the salt. 1 See answer Advertisement. - 25916372 antariusjones8519 antariusjones8519 1 week ago Chemistry High School answered Which best explains why water dissolves most salts.
Water can become so heavily attracted to a different molecule like salt NaCl that it can disrupt the attractive forces that hold the sodium and chloride in the salt molecule together and thus dissolve it. One atom gives up an electron usually a loosely held lone electron by itself in the highest occupied energy level of the donor atom. Water Doesnt Dissolve Everything Despite its name as the universal solvent there are many compounds water wont dissolve or.
These ends attract the positive and negative ions in salt and pull them apart from each other. What will happen if we go on adding more and more of salt to a fixed quantity of water. Which statement best explains waters ability to dissolve covalent compounds.
This is because HCl is a much stronger acid than water and happily sheds its proton in solutions with acidity far greater than that of water which has a neutral pH of 7. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions and the negative part of water molecules attracts the positive sodium ions. When salt is mixed with water the salt dissolves because the covalent bonds of water are stronger than the ionic bonds in the salt molecules.
When salt is mixed with water the salt dissolves because the covalent bonds of water are stronger than the ionic bonds in the salt molecules. Which is a cause of polarity in water molecules. The general rule for solvation is like dissolves like.
Also sodium hydroxide NaOH is a very strong base that would gobble up the released H ions anyway making water. Therefore when we write Na aq or Cl aq the symbol aq aqueous usually means that each ion is attracted to and surrounded by several water molecules. How sodium chloride dissolves.
Water dissolves salt because the negative part of a water molecule the oxygen part is attracted to the positive part of the salt the sodium part. The waters partial charges attract different parts of the compound making them soluble in water. Which best explains why water dissolves most salts.
Water is a very polar molecule. O Water is polar and salts form ions in solution O Water is nonpolar and salts form ions in solution O Water has the same density as salts O Water has a different density than salts. Water dissolves salt because the positive part of water molecules attracts the negative chloride ions and the negative part of water molecules attracts the positive sodium ions.
High electronegativity difference between oxygen and hydrogen. Water molecules pulling apart the ions sodium and chloride in a salt crystal. The charged ends of the water molecule attract the.
Salt sodium chloride is made from positive sodium ions bonded to negative chloride ions. The polarity of water molecules enables water to dissolve many ionically bonded substances. Salt sodium chloride is made from positive sodium ions bonded to negative chloride ions.
Water can dissolve polar covalent compounds through dipole-dipole interactions. Thus water best dissolves polar substances including ionic and polar covalent substances. The amount of a substance that.
This surrounding of sodium and chloride ions by water molecules is called hydration. Water dissolves salt by dissociating the ions in salt from each other. Which best explains why water dissolves most salts.
Chloride ions in salt are negatively charged while the sodium ions carry a positive charge. NaCl s Na aq Cl - aq Therefore dissolving salt in. When you dissolve salt in water the sodium chloride dissociates in Na ions and Cl - ions which may be written as a chemical equation.
Water dissociates salts by separating the cations and anions and forming new interactions between the water and ions. Both water and salt compounds are polar with positive and negative charges on opposite sides in the molecule. Which best explains why water dissolves most salts.

Solute And Solvent Ck 12 Foundation

Water Molecules And Their Interaction With Salt U S Geological Survey
Comments
Post a Comment